Commission Regulation (EC) No. 152/2009 establishes the analytical methods used to support official controls for enforcing the ban on the use of processed animal protein in food-producing animals.
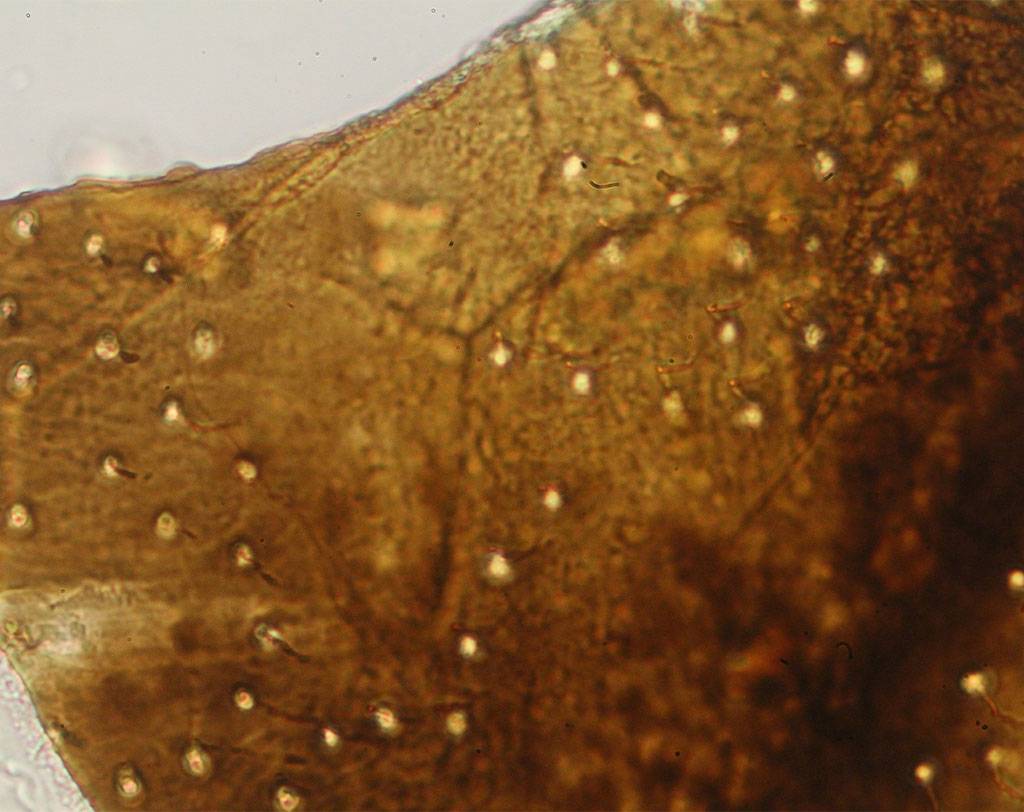
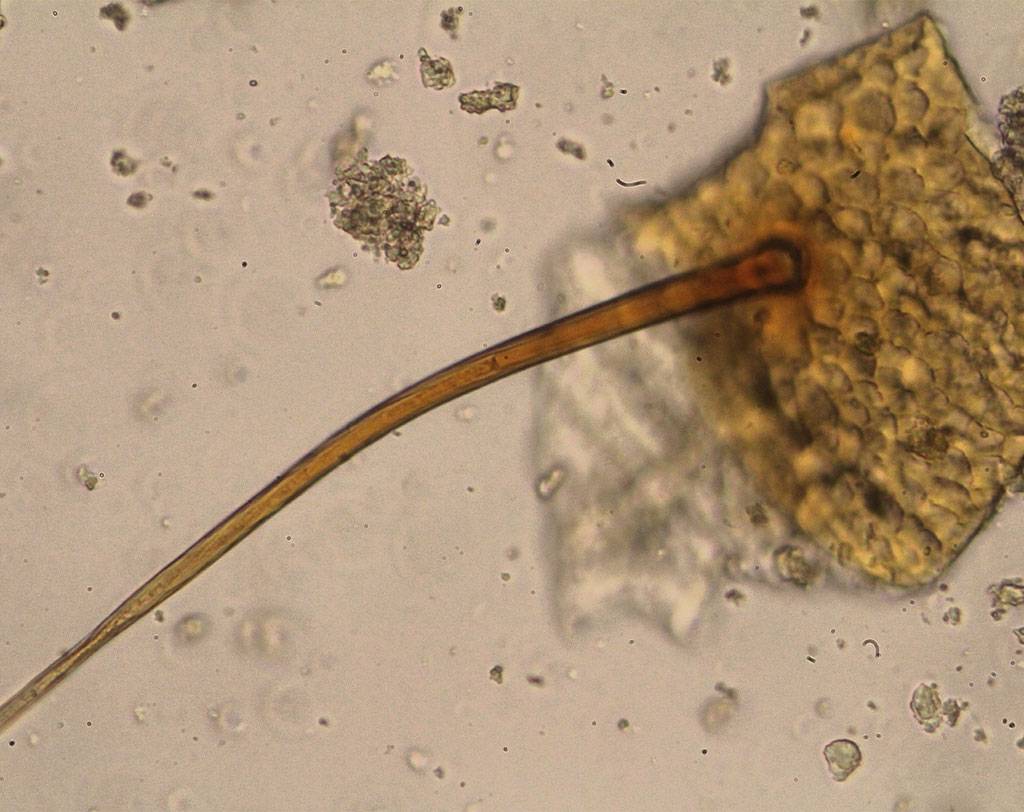
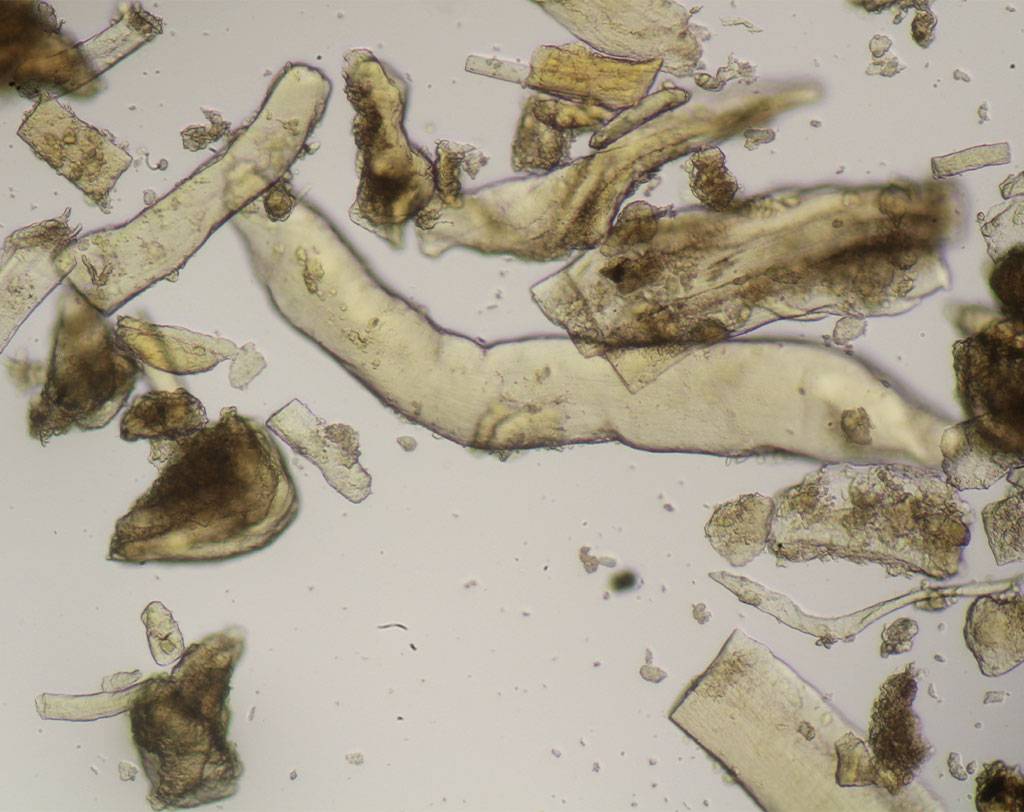
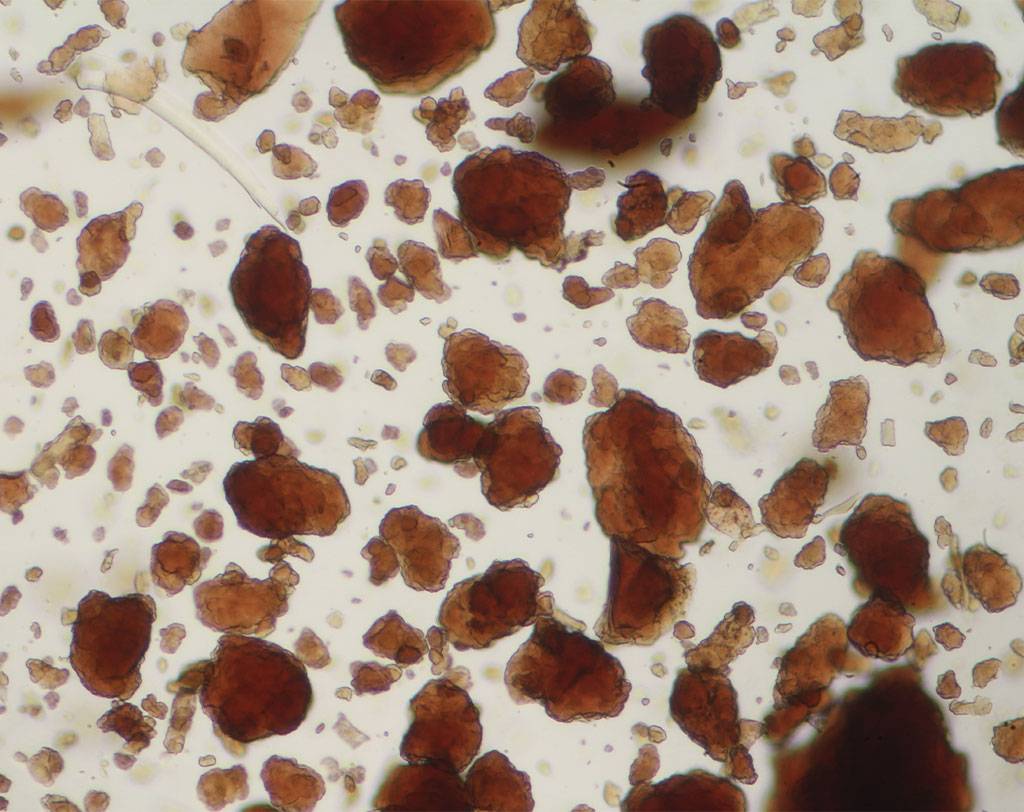
BSE (bovine spongiform encephalopathy) is a progressive neurological disease of cattle. The nature of the infectious agent that causes BSE and scrapie is unknown. Currently, the most accepted theory is that the agent is a modified form of a normal cellular protein known as a prion. To prevent the spread of BSE, a complete ban on feeding processed animal protein to any animal raised for food production was introduced on January 1, 2001. Some exceptions have since been introduced. Commission Regulation (EC) No. 152/2009 establishes the analytical methods used to support official controls for enforcing the ban on the use of processed animal protein in food-producing animals. The determination of constituents of animal origin for official feed control is carried out by light microscopy, polymerase chain reaction (PCR), or a combination of both.
Commission Implementing Regulation (EU) 2020/1560 amending Annex VI of Regulation (EC) No. 152/2009 laying down the methods of analysis for the determination of constituents of animal origin for the official control of feed changed the expression of results from “terrestrial animals” to “terrestrial vertebrates” and identifies how blood, milk globules and lactose crystals are also among the constituents of animal origin under investigation.
Regulation (EU) 2017/893 authorized the use of processed animal protein derived from farmed insects (insect PAPs) in aquaculture animal feed and in feed for pigs and poultry by Regulation (EU) 2021/1372, but it is still prohibited by Regulation (EC) No. 999/2001 of the European Parliament and of the Council in some feed, particularly in feed for ruminants. At the same time, new approvals have been issued for PAPs for pigs and poultry.
Regulation (EU) 2022/893 of June 7, 2022 amended Annex VI of Regulation (EC) No. 152/2009 by adapting the analytical protocol with the inclusion of a double sedimentation step to detect the constituents of terrestrial invertebrates, including insects, if present in feed materials, compound feed, and premixes. This additional step allows for verification of the proper implementation of the ban on the use of processed animal protein from insects in certain food-producing animal feeds.
Agrolab Alimentalia's food and feed microscopy laboratory validated and adapted the light microscopy method to include the detection of terrestrial invertebrates in the sample preparation protocol. The laboratory also participated in the first IAG Proficiency Test proposed by the microscopy laboratory EURL-AP, Center wallon de Recherches agronomiques, Gembloux - BELGIUM along with 59 other participating laboratories and identified all constituents in the tests with 100% correctness obtaining a certificate of participation in the PT. The laboratory also participated in a second Proficiency Test proposed by the National Reference Center for Animal Food Surveillance and Control (C.Re.A.A.) of the IZSPLV. All constituents in the analyzed samples were correctly detected and identified, both by the Real-Time PCR technique and by mycoroscopy, resulting in a PT certificate of participation.
The extraction efficiency of the method, coupled with the expertise of qualified technicians, ensures that reliable results are obtained. By contacting AGROLAB's technical support, you can obtain further information on the subject and agree on the analyses best suited to your needs.
Author: Dr.ssa Asma Zeiri, AGROLAB Alimentalia S.r.l.
© Pictures: AGROLAB Alimentalia S.r.l.

 Contact
Contact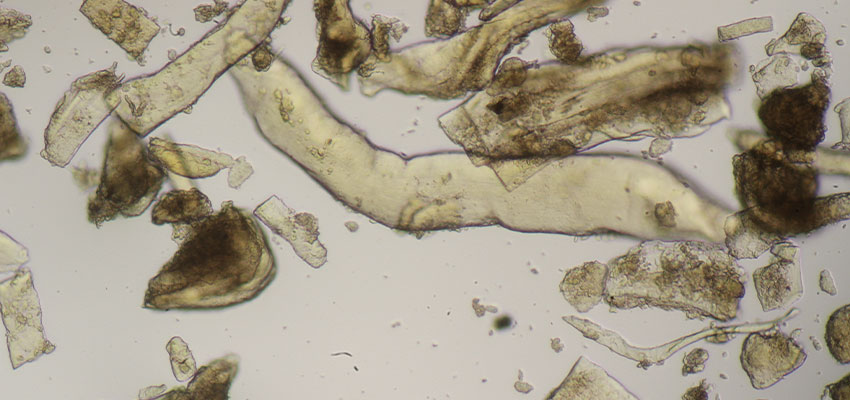





 Contact
Contact Career
Career